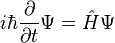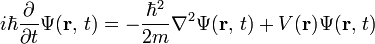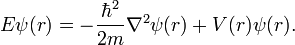members:
Miranda
Magdaraog
Paca
Paculan
Parazo
Electrons tend to arrange themselves around nuclei so that they have the lowest possible energy. They would all like to get into the lowest energy level
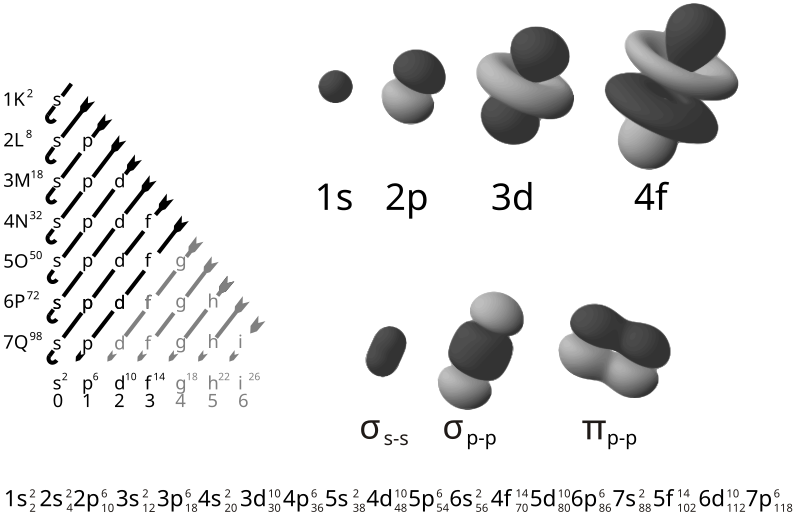
The shells are numbered outward from the Nucleus. The maximum number of electrons found in each shell can be calculated by: 2n2.gif (915 bytes) where "n" is the number of the shell.
Shell Number Maximum Number
of Electrons in the Shell
1 2 x 1 = 2
2 2 x 4 = 8
3 2 x 9 = 18
4 2 x 16 = 32
5 2 x 25 = 50
The Octet Rule:
In general, atoms are most stable when they have 8 electrons in their outer-most shell. (Octet means 8.) The exception is the first shell which is most stable with TWO electrons. If you know the Atomic Number and Mass Number of an element and the maximum number of electrons in each electron shell you can draw a diagram of the element.
Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals. For example, the first (K) shell has one subshell, called "1s"; the second (L) shell has two subshells, called "2s" and "2p"; the third shell has "3s", "3p", and "3d"; and so on.[1] The various possible subshells are shown in the following table:
Subshell label ℓ Max electrons Shells containing it Historical name
s 0 2 Every shell sharp
p 1 6 2nd shell and higher principal
d 2 10 3rd shell and higher diffuse
f 3 14 4th shell and higher fundamental
g 4 18 5th shell and higher
The electron shells are labelled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. Electrons in outer shells have higher average energy and travel farther from the nucleus than those in inner shells.
Energy Levels- Since an electron in an atom has both mass and motion, it contains two types of energy. By virtue of its motion the electron contains KINETIC ENERGY. Due to its position it also contains POTENTIAL ENERGY. The total energy contained by an electron (kinetic plus potential) is the factor which determines the radius of the electron orbit. In order for an electron to remain in this orbit, it must neither GAIN nor LOSE energy.
Orbital
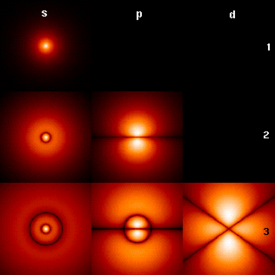
The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in each case, like a hollow ball made of rather chunky material with the nucleus at its centre.
 The orbital on the left is a 2s orbital.This is similar to a 1s orbital except that the region where there is the greatest chance of finding the electron is further from the nucleus - this is an orbital at the second energy level.
If you look carefully, you will notice that there is another region of slightly higher electron density (where the dots are thicker) nearer the nucleus. ("Electron density" is another way of talking about how likely you are to find an electron at a particular place.)
2s (and 3s, 4s, etc) electrons spend some of their time closer to the nucleus than you might expect. The effect of this is to slightly reduce the energy of electrons in s orbitals. The nearer the nucleus the electrons get, the lower their energy.
3s, 4s (etc) orbitals get progressively further from the nucleus.
The orbital on the left is a 2s orbital.This is similar to a 1s orbital except that the region where there is the greatest chance of finding the electron is further from the nucleus - this is an orbital at the second energy level.
If you look carefully, you will notice that there is another region of slightly higher electron density (where the dots are thicker) nearer the nucleus. ("Electron density" is another way of talking about how likely you are to find an electron at a particular place.)
2s (and 3s, 4s, etc) electrons spend some of their time closer to the nucleus than you might expect. The effect of this is to slightly reduce the energy of electrons in s orbitals. The nearer the nucleus the electrons get, the lower their energy.
3s, 4s (etc) orbitals get progressively further from the nucleus.
p orbitals

Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.
In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At the third level there are a total of nine orbitals altogether.
At the fourth level, as well the 4s and 4p and 4d orbitals there are an additional seven f orbitals - 16 orbitals in all. s, p, d and f orbitals are then available at all higher energy levels as well.
An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell.
Subshell- Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals. For example, the first (K) shell has one subshell, called "1s"; the second (L) shell has two subshells, called "2s" and "2p"; the third shell has "3s", "3p", and "3d"
Although it is commonly stated that all the electrons in a shell have the same energy, this is an approximation. However, the electrons in a subshell do have exactly the same level of energy, with later subshells having more energy per electron than earlier ones. This effect is great enough that the energy ranges associated with shells can overlap.