Roldan
Santiago
Sarmiento
----------
Quantum Mechanical Model is the most recent model of the atom. It is based on quantum mechanics numbers are quantum solutions to quantum equations and are used to find probable position and location of an electron in an atom.The first Principal quantum number identifies which energy level in an electron is in. There are 7 possible energy levels in an atom in the ground state (stable) the azimuthal quantum number identifies the sub-level with in the energy level where the electron is most likely to be found.
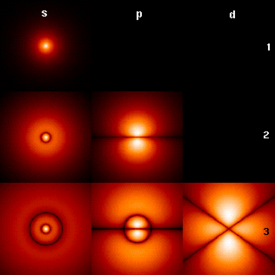 The History of Quantum Mechanics began with the 1838 discovery of cathode rays by Michael Faraday. The 1859 statement of the Black Body Radiation.
The History of Quantum Mechanics began with the 1838 discovery of cathode rays by Michael Faraday. The 1859 statement of the Black Body Radiation. Schrondinger Equation:
Time Independent Equation
Following Max Planck's quantization of light (see black body radiation), Albert Einstein interpreted Planck's quantum to be photons, particles of light, and proposed that the energy of a photon is proportional to its frequency, one of the first signs of wave–particle duality. Since energy and momentum are related in the same way as frequency and wavenumber in special relativity, it followed that the momentum p of a photon is proportional to its wavenumber k.
The Schrödinger equation
** The Schrödinger equation takes several different forms, depending on the physical situation. This section presents the equation for the general case and for the simple case encountered in many textbooks.

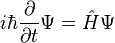
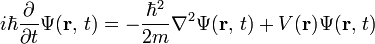
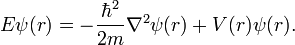
No comments:
Post a Comment